Neuro-oncologist physician-scientist Dr. Benjamin Purow describes the findings in a paper he helped author in Oncotarget titled, “Statins affect human glioblastoma and other cancers through TGF-β inhibition.”

The Behind the Study series transcribes videos of chosen researchers elaborating on their recent papers published in Oncotarget. Visit the Oncotarget YouTube channel for more insights from outstanding authors.
—
Hi, my name is Benjamin Purow. I’m a neuro-oncologist physician-scientist at the University of Virginia. I’m in the neurology department and also in the cancer center here. I’m very happy today to talk to you about our recent paper on the statins and that their primary mode of action against cancer cells is through TGF-β inhibition. So to give you a little background on what got us into this, we’re a neuro-oncology lab, as I mentioned, I’m a neuro- oncologist and we’re always interested in finding new targets, new drugs, but we’re also very interested in re-purposing existing drugs and finding better ways to apply existing drugs, new mechanisms, and combinations with drugs involving re-purposing or rescuing drugs that have been abandoned. This particular research got started in a somewhat unusual way I think. So there’s a fascinating, an underused database available online called CellMiner.
And it’s run by the NIH, the National Institutes of Health, and it makes available very detailed profiling of the NCI-60 cell line and it relates 59 cell lines and one’s probably duplicate. But at any rate, this CellMiner database involves or makes available data with gene expression profiling, microRNA expression profiling. More recently, they’ve added a lot more data with mutations and other characterization of the cell lines. But the thing that I think is most exciting is that they’ve looked at drug sensitivity with a library of over 20,000 compounds and CellMiner allows you to do very facile searches for correlations and patterns in these data. You know, when this extensive profiling of the 60 cell lines and the NCI cancer cell lines and the NCI-60. So one of the interesting ways to use this database I think, is to look for correlations in gene expression versus drug sensitivity, and to be somewhat targeted about that.
One of the things I tried to do was look at expression of established target genes for given pathways, and then look for some patterns in drug sensitivity. And one of the pathways that I did this for the TGF-β pathway, which is a well-known and well-studied in oncology. It’s an inflammatory pathway, often works in partnership or overlapping with NF-B pathway, NF-kB signaling, but TGF-β is involved in a lot of inflammatory processes, fibrosis for example, and drives a whole lot of downstream signaling. In normal cells, it acts more like a tumor suppressor, but it’s well-known as the drive or in many, many cancers, or at least a contributor to many, many cancers. The driver I would say is less well established generally in cancer but it also has some interesting pro-cancer effect, not just at the level of the cancer cells themselves, but also with the micro environment is powerfully immunosuppressive.
So, there’s a lot of interest in targeting TGF-β, but we don’t yet have any good TGF-β inhibitors in the clinic. There are some drugs dedicated TGF-V inhibitors that are in the pipeline, but there’s strong interest in getting drugs now to the clinic to block this TGF-β signaling pathway for a number of cancers including glioblastoma brain cancer, which is our main focus among those. But more broadly, this is true across lots of different cancers. So using the CellMiner database, I tried to get a sense of whether any existing drugs, known drugs, might have some relevance for the TGf-β pathway.
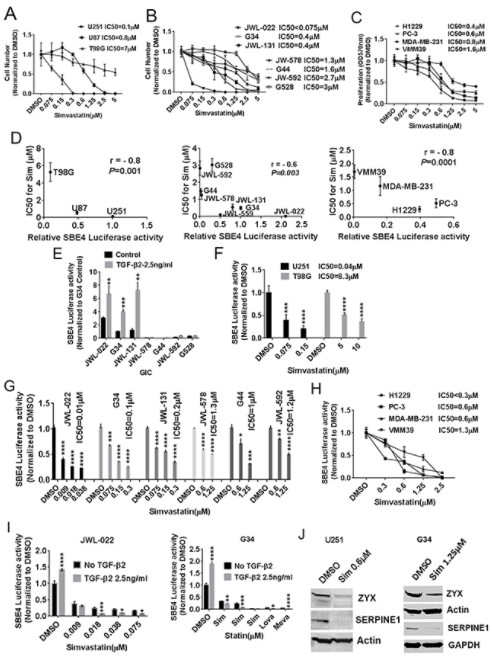
I said, I looked at expression of known TGF-β target genes and used high expression of those genes as a surrogate for high TGF-β activity, and then looked for any drug correlations, the drugs that seem to be more active against cancer lines, that seem to have high TGF-β activity, high expression of these known TGF-β target genes whose expression is driven by TGF-β activity and signaling and using a couple of good TGF-β target genes. One was called SERPINE1, there’s another one called Zyxin. Those are two classic and strong TGF-β targets that we use and looking at their expressions, the cancer cell lines that have the high expression. These TGF-β target genes were much more sensitive if you looked at the drug sensitivities to class of drugs called the statins and the statins are used ubiquitously out in the clinic.
There are a host of these approved FDA approved statin drugs, which have been out there for decades as cholesterol lowering drugs. These enzymes inhibit potently an enzyme called HMG-CoA reductase, which is involved in cholesterol synthesis in our bodies. So the statins have been used ubiquitously for ages. They’re among the most highly prescribed drugs in the whole world as cholesterol lowering agents. So, but, we made this interesting finding with CellMiner that cancer cell lines with seemingly high TGF-β activity using expression of these TGF-β targets as a surrogate marker, all the standards that were in the library seemed to correlate.
It was sort of an amazing degree of correlation. We did some statistics on how to get p-values to characterize these how significant these correlations were so we got the most ridiculous p-values I’ve ever seen anywhere. Things like 10 to the minus-20, you know what I mean? So we got fairly absurd correlations of statin sensitivity with expression in cancer lines of TGF-β target genes. So naturally we wanted to follow this up and gave us the idea that, well, firstly, that the statins were more active against cancer lines with high TGF-β, that in itself is interesting. But it also suggests to us, well, maybe that’s because statins actually inhibit TGF-β. Now the role of the statins and oncology has been kind of a confusing one. There are some interesting epidemiologic studies in different cancers suggesting that there may be better survival patients with certain cancers when they’re on statins.
There are a few papers in the opposite direction that patients on statins tend to do a little worse, or that those cancers are a little more common in patients on statins. So, but it’s been really a mess and people have not had a good sense of how the statins might plug in to oncology. There have been a number of papers suggesting that the statins have some direct activity against cancer cells and a few different mechanisms have been positive for that. So it’s been thought that the statins might do some modest inhibition of this NF-kB pathway, which is a sister pathway to TGF-β, but not a lot of attention was given to that. And the statins don’t seem to be particularly good against NF-kB actually, the statins do act to some degree is prenylation inhibitors by blocking availability of upstream substrates for prenylation.
But, there really wasn’t a solid sense in the cancer literature of what the statins might be doing against cancer cells and is there a primary mechanism for that. So we started digging into the statins as potential TGF-β inhibitors. And so, one of the things we looked at was whether, trying to confirm whether cancer cell lines that had higher TGF-β activity were indeed more sensitive to the Statins. And we did see, we did confirm that we did see correlations that looking at with glioblastoma or GBM cell lines, that higher activity, higher TGF-β activity did correlate with sensitivity to the statins. And we focus mostly on simvastatin where it’s Zocor is the trade name. This is a widely used statin. It’s the one that probably based on past research has the best blood brain barrier penetration. So it gets into the brain the best.
So for our purposes, with a focus on glioblastoma, we were interested in focusing on simvastatin, but we showed the same things with other statins. So this was not at all specific to just in the statin. So we did show a correlation it’s more cell lines of higher TGF-β activity to statin and sensitivity. And we looked at TGF-β activity in a couple of ways. We in the paper used a whole lot, a well-established reporter plasmid that uses the luciferase expression driven by SMAD3 binding elements as a good surrogate reporter for TGF-β activity. And we use that reagent throughout the paper, as well as looking at expression of target teams like Turpin U1 and zyxin. So, that was one of the things we showed was confirming that correlation. Along those lines, we also showed that you could manipulate the level of TGF-β activity in these cells and affect their sensitivity to the statins.
So for example, if you gave exogenous TGF-β is bumped up the level of TGF-β activity, you actually sensitize them to the statin. So we thought that was a nice bit of support showing the TGF-β activity. It was a controller for how sensitive these cancer cells wore to the statins. And I should mention that we mostly focused on GBM in the paper, but we did show the same basic principles for other cancers as well. So we think this is broadly applicable across cancer and the TGF-β activity does regulate how sensitive cancer cells are to the statins. Now we wanted to check too, as I said, whether the statins could actually inhibit TGF-β cells, and we found that they were actually quite potent TGF-β inhibitors, and importantly, this was at clinically achievable levels. So, or physiologically relevant concentrations of the sentence.
So for example, simvastatin. It’s standard dosing and a patient you can use 20 or 40 milligrams a day of simvastatin in the patient Zocor. Blood levels can reach something like 700 nanomolar or potentially 500, 700 nanomolar. And we did see activity of the stands against TGF-beta in concentrations at that range. Then one important thing to mention though, is that there was some variability in how sensitive different cancer cell lines were to the satins. So looking at that reporter plasma that I mentioned and downstream targets of TGF-β, we saw that the statins were able to potently and strongly reduce TGF-β activity, but the concentrations in which that happened differed across different cell lines. And we noted that cell lines that tended to be a little more mesenchymal in their phenotype, and you’ll see that mesenchymal phenotype pop-up and all kinds of cancers.
There’s the so-called epithelial to mesenchymal transition, EMT, and that’s a major issue in lots of different epithelial cancers, breast, lung, prostate, et cetera. They tend to have an epithelial phenotype most of the time, but it’s EMT can occur drive them to more mesenchymal phenotype TGF-β fittingly is known as one of the major drivers of EMT and lots of cancers. So it makes sense that the more mesenchymal cancers across all different cancers and this includes GBM, it makes sense that the more mesenchymal ones might have greater TGF-β activity and dependency. And we found that those cell lines tended to be sensitive to the statins at lower concentrations and typically more physiologically relevant concentrations. So mechanistically we did come up with a mechanism by which the statins inhibited TGF-β, that being said, there may be other mechanisms out there.
Basically for that mechanism, we put two and two together about some things that were in the literature already. I know before our paper, there really hadn’t been papers that had shown what we found, that the Statins were strong TGF-β inhibitors in cancer cells. And then in fact, it was their primary mechanism. There were some hints in the literature that statins might be effecting some downstream mediators of TGF-β, for example, but basically everything we showed was new. However, the mechanism was a bit easy based on some prior things that had been shown. And there was a phosphorylation of SMAD3 which is a critical TGF-β mediator. And we found that the statins affected the enzymes that did this phosphorylation and therefore was affecting SMAD3 activity. So we think we have a reasonable mechanism to explain how statins affect TGF-β activity, but there are potentially other plausible things that might be further contributing to statin’s effects on TGF-β activity.
Now, one interesting thing is that, we did look at a couple of commercially available TGF-β inhibitors, including one drug that is in clinical trials as a TGF-β inhibitor. And we were a bit shocked to see that the statins actually did very well against the dedicated TGF-β inhibitors when they were compared for potency and a level of TGF-β inhibition. In fact, the statins tended to outperform the TGF, the dedicated TGF-β inhibitors in the pipeline. Now statins are of course, dirty drugs. They do other things, they lower cholesterol, they gets in prenylation inhibition, and by themselves, we’re not really advocating for the statins as solo cancer therapies, but, you knowing that they’re more active against cancer cell lines with higher TGF-β activity, we think is very important. And we think they’re particularly relevant against mesenchymal cancer cell lines.
There is one paper that also alludes to this in the literature about, maybe the statins being more relevant from mesynchemal cancer lines. And we think we have a good explanation for this, where the TGF-β inhibition by the statins. In terms of where do we go from here going forward? Well, we think it’s very important to know that the statins are strong TGF-β inhibitors. We saw that it wasn’t just in normal cancer cells or normal liver seemed to show signs that TGF-β inhibition as well. So we think the implications are potentially pretty broad. We think that this can really help guide combination therapies of statins plus other drugs. There’s certain other drugs where one of the rapid resistance mechanisms is an EMT and it may often be TGF-β driven, adding a statin into one of those drugs we might block that resistance mechanism.
So we’ve been working now with combination therapies of statins plus other drugs. And there are a lot of great synergistic interactions of statins plus other agents. And we think that the TGF-β inhibition by the statins is really the critical mechanism there. And we think this may be important to in other settings too. I mean, there’ve been hints that the statins may somewhat prolong lifespan and do some other exciting things. I mean, obviously they’re used as cholesterol lowering drugs, there have been hints of anti inflammatory activity, but we think inhibiting TGF-β might explain a bunch of the beneficial activity of the statins actually. Some of their anti-fibrotic activity.
TGF-β is a big driver of fibrosis in inflammation. TGF-β is a major mediator of inflammation. So we think that showing that the statins are potent TGF-β inhibitors, will help guide their use in drug combinations against cancer, but also have significant implications outside of cancer and there’s all this interest in dedicated TGF-β inhibitors and clinical trials being done, but we’ve got these really quite good TGF-β inhibitors already in the clinic with the status. So we’re excited about some of the directions moving forward and some of the ways that this can be used in oncology and outside as well.
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.