Researchers used mathematical modeling to investigate mechanisms that drive the elusive phenomenon of cancer cell resistance to epithelial-mesenchymal transition (EMT).
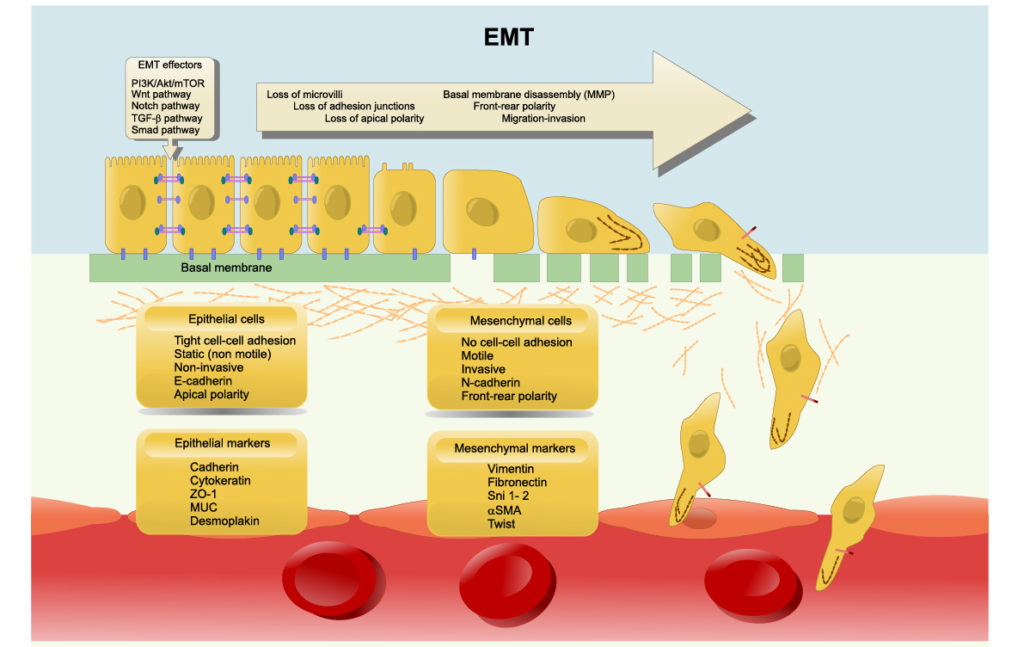
Cancer cells have been known to use sagacious methods of evading apoptosis and mysteriously overcoming powerful anti-cancer therapies. One such method of evasion has recently been identified as the process of epithelial-mesenchymal transition (EMT) and its reverse process, mesenchymal-epithelial transition (MET). These transitions enable epithelial cells (structural/fixed) to gain mesenchymal cell (differentiating/mobile) functions, and vice versa. Researchers believe that epithelial-mesenchymal plasticity (EMP) allows cancers to become therapy resistant, determines cancer aggressiveness and allows metastatic cancer to mobilize and spread.
“Such dynamic and reversible switching can help tumor cells to overcome various challenges during disease progression such as anoikis [6], and assaults by the immune system [7].”
These processes and their characterization in cancer have been studied, however, questions remain about their molecular determinants and degree of reversibility, or irreversibility, in different cell populations and environments. To further elucidate EMT, researchers from Rice University, Northeastern University and the Indian Institute of Science used mechanistic mathematical models to identify possible mechanisms that may drive EMT response to an EMT-inducing signal in a given isogenic cell population. Their paper was published by Oncotarget in 2020, and entitled, “Epigenetic feedback and stochastic partitioning during cell division can drive resistance to EMT.”
EMT/MET Reversibility/Irreversibility
In the introduction of this paper, the authors discuss results from previous research about the reversibility and irreversibility of EMT/MET. EMT can be triggered by various EMT-inducing external signals, such as TGFβ or by adjusting the levels of EMT-specific transcription factors (EMT-TFs). They report that, in cells stimulated over shorter durations (between two and six days), cells may revert back to an epithelial state after withdrawal of the signal/stimulus. They also explain that cells that have been stimulated over longer durations (10+ days) may render EMT irreversible and to become “locked” in a mesenchymal state.
Researchers suspect the existence of a “tipping point” after continued signal/stimulus exposure is what results in irreversible EMT. Multiple mechanisms have been proposed as responsible for this tipping point, including epigenetic alterations and self-stabilizing feedback loops in regulatory circuits. However, there remains a need for studies to investigate the mechanistic basis that causes epithelial cells to be resistant to undergoing EMT, or the irreversibility of MET.
“Some sporadic observations about the resistance of epithelial cells to undergo EMT have been reported [14, 24], but a causative mechanistic understanding still remains elusive.”
The Study
To investigate the mechanisms that enable the irreversibility of MET, or lack of EMP, the researchers in this study used mechanism-based mathematical modeling. Their experimental observations indicated that a global epigenetic program limiting the action of ZEB1 was found to underlie epithelial trait retention in cells exposed to persistent Twist1 activation for 21 days. They demonstrated a possible underlying mechanism by which GRHL2 overexpression can resist EMT. Importantly, the researchers found that, from a single isogenic cell population, two subpopulations of cells emerged and responded differently to the EMT-signalling.
“Here, we propose two independent mechanism[s] that may explain the resistance of epithelial tumor cells to undergo EMT: 1) epigenetic feedback mediated via GRHL2—an MET-inducing transcription factor (MET-TF) [25–27]; and 2) stochastic partitioning of parent cell biomolecules among the daughter cells at the time of cell division [28–30].”
Aside from epigenetic mediation involving GRHL2, the researchers believe varying EMT-signal responses within isogenic cell populations are caused by stochastic partitioning of molecules during cell division. The researcher described this phenomenon as a type of incongruent “noise” that takes place when cells divide.
“Such noise in the distribution of molecules may affect cell-fate and drive non-genetic heterogeneity [28–30], leading to different phenotypic distributions in terms of EMT [3].”
Conclusion
The team concluded that MET should not only be considered the reverse process of EMT, as important and distinct processes may be involved in both EMT and MET transformations. The authors are forthcoming about limitations in their study—indicating that a more detailed molecular mechanism-based epigenetic model would provide better insights into EMT. They also note that they did not consider spatial effects in their model, where more dense or spread out cell populations and access to signal strength, nutrients and oxygen may change outcomes.
“Future efforts should decode the molecular mechanisms of any such epigenetic feedback of GRHL2 on ZEB1 expression as well as track the distribution of molecules during cell divisions happening while cells are being induced to undergo EMT/MET.”
Click here to read the full research paper published by Oncotarget.
Watch, read or listen to an Oncotarget Interview with Drs. Herbert Levine and Mohit Kumar Jolly as they discuss this paper.
YOU MAY ALSO LIKE: More Oncotarget Videos on LabTube
—
Oncotarget is a unique platform designed to house scientific studies in a journal format that is available for anyone to read—without a paywall making access more difficult. This means information that has the potential to benefit our societies from the inside out can be shared with friends, neighbors, colleagues, and other researchers, far and wide.
For media inquiries, please contact media@impactjournals.com.




